Microbiota according to gastric topography in patients with low or high risk of gastric cancer in Nariño, Colombia
Abstract
Introduction: Inflammation in the gastric antrum caused by Helicobacter pylori increases the risk of duodenal ulcer while inflammation in the body generates atrophic gastritis and increased risk of gastric cancer. These inflammatory responses according to gastric topography could be explained by the composition of the gastric microbiota associated with H. pylori.
Objective: To identify and compare the microbiota of the gastric antrum and body of individuals from two populations, one with high risk and one with low risk of gastric cancer from Nariño, Colombia.
Materials and methods: Biopsies of the gastric antrum and body of patients with non-atrophic gastritis or metaplastic atrophic gastritis were included. The microbiota was defined by sequencing the 16S rRNA gene, V3-V4 region, (illumina-MiSeq™). The operational taxonomic units were classified using the BLASTn and RDPII databases. The differences among microbial populations were evaluated with the PERMANOVA and multivariate analyses.
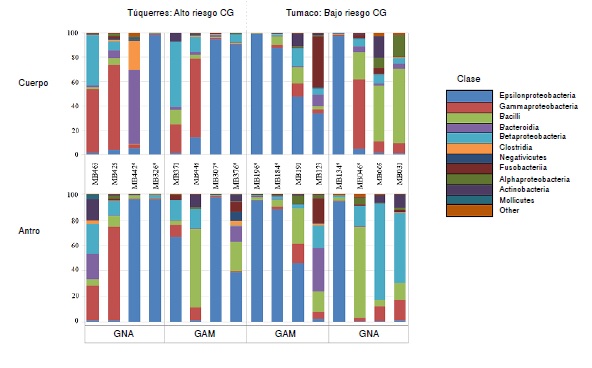
Results: The Epsilonproteobacteria class represented by H. pylori was more abundant in the antrum and body biopsies of individuals with metaplastic atrophic gastritis (>50%) while in individuals with non-atrophic gastritis it was 20 % and had greater metagenomic diversity. Helicobacter pylori infection significantly decreases the metagenomic diversity of the gastric antrum (p=0.005) compared to that of the body.
Conclusions: The bacterial groups involved in the dysbiosis can colonize both topographic regions of the stomach, regardless of the sectorized inflammation responses. Helicobacter pylori infection associated with the gastric microbiota is related to its localization in the stomach, the type of lesion, and the population at risk of gastric cancer, which suggests its importance in microbial dysbiosis and gastric disease.
Downloads
References
Sipponen P, Kekki M, Seppala K, Siurala M. The relationships between chronic gastritis and gastric acid secretion. Aliment Pharmacol Ther. 1996;10:103-18. https://doi.org/10.1046/j.1365-2036.1996.22164011.x
Kong YJ, Yi HG, Dai JC, Wei MX. Histological changes of gastric mucosa after Helicobacter pylori eradication: A systematic review and meta-analysis. World J Gastroenterol. 2014;20:5903-11. https://doi.org/10.3748/wjg.v20.i19.5903
Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-9. https://doi.org/10.1056/NEJMoa001999
Camargo MC, Anderson WF, King JB, Correa P, Thomas C, Rosenberg P, et al. Divergent trends for gastric cancer incidence by anatomical subsite in US adults. Gut. 2011;60:1644-9. https://doi.org/10.1136/gut.2010.236737
Sheh A, Fox J. The role of the gastrointestinal microbiome in Helicobacter pylori pathogenesis. Gut Microbes. 2013;4:505-31. https://doi.org/10.4161/gmic.26205
Correa P. Human gastric carcinogenesis: A multistep and multifactorial process--first American Cancer Society award lecture on cancer epidemiology and prevention. Cancer Res. 1992;52:6735-40.
Brawner KM, Morrow CD, Smith PD. Gastric microbiome and gastric cancer. Cancer J. 2014;20:211-6. https://doi.org/10.1097/PPO.0000000000000043
Nardone G, Compare D. The human gastric microbiota: Is it time to rethink the pathogenesis of stomach diseases? United European Gastroenterol J. 2015;3:255-60. https://doi.org/10.1177/2050640614566846
Yang I, Woltemate S, Piazuelo MB, Bravo LE, Yépez MC, Romero-Gallo J, et al. Different gastric microbiota compositions in two human populations with high and low gastric cancer risk in Colombia. Sci Rep. 2016;6:18594. https://doi.org/10.1038/srep18594
Avilés F, Vázquez F, Medrano R, Mantilla A, Torres J. Stomach microbiota composition varies between patients with non-atrophic gastritis and patients with intestinal type of gastric cancer. Sci Rep. 2014;4:4202. https://doi.org/10.1038/srep04202
Correa P, Cuello C, Duque E, Burbano LC, García FT, Bolaños O, et al. Gastric cancer in Colombia. III. Natural history of precursor lesions. J Natl Cancer Inst. 1996;57:1027-35.
Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis. Am J Surg Pathol. 1996;20:1116-81.
Figueroa M, Cortés A, Pazos A, Bravo L. Sensibilidad in vitro a amoxicilina y claritromicina de Helicobacter pylori obtenido de biopsias gástricas de pacientes en zona de bajo riesgo para cáncer gástrico. Biomédica. 2012;32:32-42. https://doi.org/10.1590/S0120-41572012000100005
Takahashi S, Tomita J, Nishioka K, Hisada T, Nishijima M. Development of a prokaryotic universal primer for simultaneous analysis of bacteria and archaea using next-generation sequencing. PLoS One. 2014;9:e105592. https://doi.org/10.1371/journal.pone.0105592
Dowd SE, Callaway TR, Wolcott RD, Sun Y, McKeehan T, Hagevoort RG, et al. Evaluation of the bacterial diversity in the feces of cattle using 16S rDNA bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP). BMC Microbiol. 2008;8:125. https://doi.org/10.1186/1471-2180-8-125
Rodríguez TM, Fornaciari G, Luciani S, Dowd SE, Toranzos GA, Marota I, et al. Gut microbiome of an 11th Century A.D. Pre-Columbian Andean mummy. PLoS One. 2015;10:e0138135. https://doi.org/10.1371/journal.pone.0138135
Maidak BL, Cole JR, Lilburn TG, Parker CT, Saxman PR, Farris RJ, et al. The RDP-II (Ribosomal Database Project). Nucleic Acids Res. 2001;29:73-4. https://doi.org/10.1093/nar/gkt1244
Caporaso J, Kuczynski J, Stombaugh J, Bittinger K, Bushman F, Costello E, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335-6. https://doi.org/10.1038/nmeth.f.303
Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001;26:32-46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x
Ramette A. Multivariate analyses in microbial ecology. FEMS Microbiol Ecol. 2007;62:142-60. https://doi.org/10.1111/j.1574-6941.2007.00375.x
Hammer Ø, Harper D, Ryan PD. Paleontological statistics software package for education and data analysis. Palaeontol Electron. 2001;4: 9-18.
Correa P, Piazuelo MB. Helicobacter pylori infection and gastric adenocarcinoma. US Gastroenterol Hepatol Rev. 2011;7:59-64.
Koskenpato J, Färkkilä M, Sipponen P. Helicobacter pylori and different topographic types of gastritis: Treatment response after successful eradication therapy in functional dyspepsia. Scand J Gastroenterol. 2002;37:778-84. https://doi.org/10.1080/gas.37.7.778.784
Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60-9. https://doi.org/10.1172/JCI30111
Rolig AS, Shanks J, Carter JE, Ottemann KM. Helicobacter pylori requires TlpD-driven chemotaxis to proliferate in the antrum. Infect Immun. 2012;80:3713-20. https://doi.org/10.1128/IAI.00407-12
Rieder G, Merchant JL, Haas R. Helicobacter pylori cag-type IV secretion system facilitates corpus colonization to induce precancerous conditions in Mongolian gerbils. Gastroenterology. 2005;128:1229-42. https://doi.org/10.1053/j.gastro.2005.02.064
Castaño N, Goh, KL, Fock KM, Mitchell HM, Kaakoush NO. Dysbiosis of the microbiome in gastric carcinogenesis. Sci Rep. 2017;7:15957. https://doi.org/10.1038/s41598-017-16289-2
Fragkiadakis K, Ioannou P, Barbounakis E, Samonis G. Intra-abdominal abscesses by Lactococcus lactis ssp cremoris in an immunocompetent adult with severe periodontitis and pernicious anemia. IDCases. 2017;7:27-9. https://doi.org/10.1016/j.idcr.2016.12.001
Haas R, Smith J, Rocher V, Nadkarni S, Montero T, D’Acquisto, F, et al. Lactate regulates metabolic and pro-inflammatory circuits in control of T cell migration and effector functions. PLoS Biol. 2015;13:e1002202. https://doi.org/10.1371/journal.pbio.1002202
Romero S, Moreno M, Prado H, Sánchez F. Lactate contribution to the tumor microenvironment: Mechanisms, effects on immune cells and therapeutic relevance. Front Immunol. 2016;7:52. https://doi.org/10.3389/fimmu.2016.00052.ghjg
Vitetta L, Coulson S, Thomsen M, Nguyen T, Hall S. Probiotics, D–Lactic acidosis, oxidative stress and strain specificity. Gut Microbes. 2017;8:311-322. https://doi.org/10.1080/19490976.2017.1279379.gjh
Engstrand L, Lindberg M. Helicobacter pylori and the gastric microbiota. Best Pract Res Clin Gastroenterol. 2013;27:39-45. https://doi.org/10.1016/j.bpg.2013.03.016
Li XX, Wong GL, To KF, Wong VW, Lai LH, Chow DK, et al. Bacterial microbiota profiling in gastritis without Helicobacter pylori Infection or non-steroidal anti-inflammatory drug use. PLoS One. 2009;4:e7985. https://doi.org/10.1371/journal.pone.0007985
Maughan H, Redfield RJ. Tracing the evolution of competence in Haemophilus influenzae. PLoS One. 2009;4:e5854. https://doi.org/10.1371/journal.pone.0005854
Alarcón T, Llorca L, Pérez G. Impact of the microbiota and gastric disease development by Helicobacter pylori. Curr Top Microbiol Immunol. 2017;400:253-75. https://doi.org/10.1007/978-3-319-50520-6_11
Yu G, Torres J, Hu N, Medrano R, Herrera R, Humphrys MS, et al. Molecular characterization of the human stomach microbiota in gastric cancer patients. Front Cell Infect Microbiol. 2017;7:302. https://doi.org/10.3389/fcimb.2017.00302
Kocsmár É, Szirtes I, Kramer Z, Szijártó A, Bene L, Buzás GM, et al. Sensitivity of Helicobacter pylori detection by Giemsa staining is poor in comparison with immunohistochemistry and fluorescent in situ hybridization and strongly depends on inflammatory activity. Helicobacter. 2017;22:e12387. https://doi.org/10.1111/hel.12387
Wang YK, Kuo FC, Liu CJ, Wu MC, Shih HY, Wang SS, et al. Diagnosis of Helicobacter pylori infection: Current options and developments. World J Gastroenterol . 2015;21:11221-35. https://doi.org/10.3748/wjg.v21.i40.11221
Wang LL, Yu XJ, Zhan SH, Jia SJ, Tian ZB, Dong QJ. Participation of microbiota in the development of gastric cancer. World J Gastroenterol. 2014;20:4948-52. https://doi.org/10.3748/wjg.v20.i17.4948
Croxen MA, Sisson G, Melano R, Hoffman PS. The Helicobacter pylori chemotaxis receptor TlpB (HP0103) is required for pH taxis and for colonization of the gastric mucosa. J Bacteriol. 2006;188:2656-65. https://doi.org/10.1128/JB.188.7.2656-2665.2006
Li TH, Qin Y, Sham PC, Lau KS, Chu K-M, Leung WK. Alterations in gastric microbiota after H. pylori eradication and in different histological stages of gastric carcinogenesis. Sci Rep. 2017;7:44935. https://doi.org/10.1038/srep44935
Wang L, Zhou J, Xin Y, Geng C, Tian Z, Yu X, et al. Bacterial overgrowth and diversification of microbiota in gastric cancer. Eur J Gastroenterol Hepatol. 2016;28:261-6. https://doi.org/10.1097/MEG.0000000000000542
Das A, Pereira V, Saxena S, Ghosh TS, Anbumani D, Bag S, et al. Gastric microbiome of Indian patients with Helicobacter pylori infection, and their interaction networks. Sci Rep. 2017;7:15438. https://doi.org/10.1038/s41598-017-15510-6
Kong YJ, Yi HG, Dai JC, Wei MX. Histological changes of gastric mucosa after Helicobacter pylori eradication: A systematic review and meta-analysis. World J Gastroenterol. 2014;20:5903-11. https://doi.org/10.3748/wjg.v20.i19.5903
Dore MP, Cipolli A, Ruggiu MW, Manca A, Bassotti G, Pes GM. Helicobacter pylori eradication may influence timing of endoscopic surveillance for gastric cancer in patients with gastric precancerous lesions: A retrospective study. Medicine (Baltimore). 2018;97:e9734. https://doi.org/10.1097/MD.0000000000009734
Some similar items:
- Oscar F. Herrán, María F. Ardila, Martha P. Rojas, Gustavo A. Hernández, Design of dietary questionnaires to study the relationships between diet and cancer prevalence in Colombia , Biomedica: Vol. 30 No. 1 (2010)
- Juan Carlos Cataño, Eaton-Lambert myasthenic syndrome , Biomedica: Vol. 30 No. 3 (2010)
- Juan Carlos Herrera, Luis Fernando Isaza, José Luis Ramírez, Gonzalo Vásquez, Carlos Mario Muñetón, Detection of chromosome 17 aneuplody and TP53 gene deletion in a broad variety of solid tumors by dual-color fluorescence in situ hybridization (FISH) , Biomedica: Vol. 30 No. 3 (2010)
- Yaliana Tafurt-Cardona, Leidy D. Jaramillo-Ruiz, Wilson Muñoz-Ordóñez, Sulma L. Muñoz-Benítez, Carlos H. Sierra-Torres, High frequency of chromosome aberrations observed in lymphocytes in postmenopausal obese women , Biomedica: Vol. 32 No. 3 (2012)
- Ricardo Cendales, Constanza Pardo, Claudia Uribe, Guillermo López, María Clara Yépez, Luis Eduardo Bravo, Data quality at population-based cancer registries in Colombia , Biomedica: Vol. 32 No. 4 (2012)
- Sonia Isabel Cuervo, Ricardo Sánchez, Julio César Gómez-Rincón, Cielo Almenares, Juan Pablo Osorio, María José Vargas, Behavior of carbapenemase-producing Klebsiella pneumoniae cases in cancer patients at a third level hospital in Bogotá, D.C. , Biomedica: Vol. 34 (2014): Abril, Suplemento 1, Resistencia bacteriana
- Clara Andrea Rincón-Cortés, Edgar Antonio Reyes-Montaño, Nohora Angélica Vega-Castro, Partial purification of peptides present in the Tityus macrochirus (Buthidae) scorpion venom and preliminary assessment of their cytotoxicity , Biomedica: Vol. 37 No. 2 (2017)
- Esther de Vries, María Ximena Meneses, Marion Piñeros, Years of life lost as a measure of cancer burden in Colombia, 1997-2012 , Biomedica: Vol. 36 No. 4 (2016)
- Raúl Isaías-Tizapa, Erika Acosta, Arvey Tacuba-Saavedra, Miguel Mendoza-Catalán, Napoleón Navarro-Tito, Leptin induced Hic-5 expression and actin puncta formation by the FAK/Src-dependent pathway in MCF10A mammary epithelial cells. , Biomedica: Vol. 39 No. 3 (2019)
- Arley Gómez , Microbiome, health and illnesses: probiotics, prebiotics and synbiotics , Biomedica: Vol. 39 No. 4 (2019)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























