Gene expression profiles of ERG11, MDR1 and AFR1 in Cryptococcus neoformans var.grubbi from HIV patients
Abstract
Introduction: Fluconazole is the most used antifungal drug for prevention and treatment of Cryptococcus spp. infections, the etiological agent of cryptococcosis. Resistance to fluconazole among Cryptococcus neoformans isolates can lead to treatment failure and generate relapses.
Objective: To evaluate the expression profiles of the AFR1, MDR1 and ERG11 genes in C. neoformans var. grubii clinical isolates during the in vitro response to fluconazole induction.
Materials and methods: Fourteen C. neoformans var. grubii isolates recovered from HIV patients were studied, in which 6 showed sensitivities to fluconazole and 8 decreased sensitivity. The expression levels of ERG11, AFR1 and MDR1 genes were determined by real-time PCR from extracted mRNA.
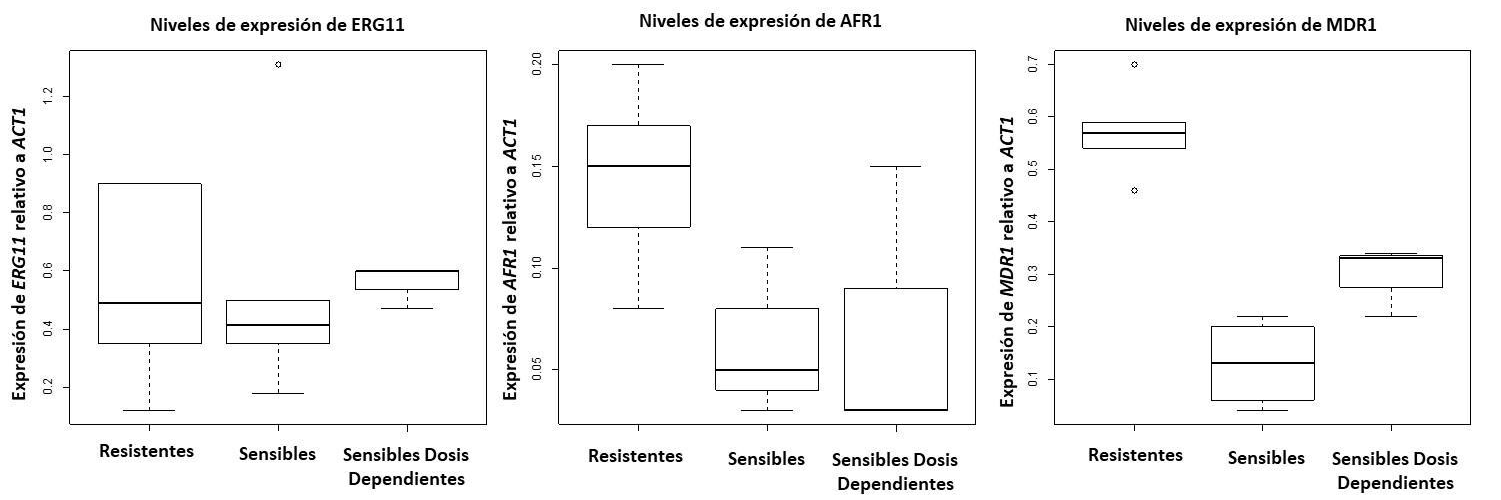
Results: AFR1 and MDR1 genes from C. neoformans var. grubii were overexpressed in fluconazole resistant isolates, whereas ERG11 maintains homogeneous expression in all
the evaluated resistance phenotypes of C. neoformans var. grubii isolates.
Conclusions: The overexpression of AFR1 and MDR1 genes, which codify for efflux pumps, contributes to fluconazole resistance in the studied isolates. However, the resistance patterns in this fungus and the relapse cases in HIV patients cannot be attributed solely to the exposure to the drug. Heteroresistance and the emerging resistance (resistance through other ERG genes), might be other mechanisms involved in this phenomenon, which must be studied in these isolations.
Downloads
References
Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2010;50:291-322. https://doi.org/10.1086/649858
Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, et al. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect Dis. 2017;17:873-81. https://doi.org/10.1016/S1473-3099(17)30243-8
Escandón P, Lizarazo J, Agudelo CI, Castañeda E. Cryptococcosis in Colombia: Compilation and analysis of data from laboratory-based surveillance. J Fungi (Basel). 2018;4(1). https://doi.org/10.3390/jof4010032
Kwon-Chung KJ, Bennett JE, Wickes BL, Meyer W, Cuomo CA, Wollenburg KR, et al. The case for adopting the “Species Complex” nomenclature for the etiologic agents of cryptococcosis. mSphere. 2017;2:e00357-16. https://doi.org/10.1128/mSphere.00357-16
Hagen F, Khayhan K, Theelen B, Kolecka A, Polacheck I, Sionov E, et al. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet Biol. 2015;78:16-48. https://doi.org/10.1016/j.fgb.2015.02.009
Idnurm A, Lin X. Rising to the challenge of multiple Cryptococcus species and the diseases they cause. Fungal Genet Biol. 2015;78:1-6. https://doi.org/10.1016/j.fgb.2015.05.002
Serna-Espinosa BN, Guzmán-Sanabria D, Forero-Castro M, Escandón P, Sánchez-Quitian ZA. Environmental Status of Cryptococcus neoformans and Cryptococcus gattii in Colombia. J Fungi (Basel). 2021;7. https://doi.org/10.3390/jof7060410
Gushiken AC, Saharia KK, Baddley JW. Cryptococcosis. Infect Dis Clin North Am. 2021;35:493-514. https://doi.org/10.1016/j.idc.2021.03.012
World Health Organization. Guidelines for the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and ahildren: Supplement to the 2016 Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva: World Health Organization; 2018. p. 51.
Bicanic T, Harrison T, Niepieklo A, Dyakopu N, Meintjes G. Symptomatic relapse of HIV-associated cryptococcal meningitis after initial fluconazole monotherapy: The role of fluconazole resistance and immune reconstitution. Clin Infect Dis. 2006;43:1069-70. https://doi.org/10.1086/507895
Maziarz EK, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2016;30:179-206. https://doi.org/10.1016/j.idc.2015.10.006
Agudelo CA, Muñoz C, Ramírez A, Tobón AM, de Bedout Bact C, Cano LE, et al. Response to therapy in patients with cryptococcosis and AIDS: Association with in vitro susceptibility to fluconazole. Rev Iberoam Micol. 2015;32:214-20. https://doi.org/10.1016/j.riam.2014.07.006
Cheong JWS, McCormack J. Fluconazole resistance in cryptococcal disease: Emerging or intrinsic? Med Mycol. 2013;51:261-9. https://doi.org/10.3109/13693786.2012.715763
Basso LR, Gast CE, Bruzual I, Wong B. Identification and properties of plasma membrane azole efflux pumps from the pathogenic fungi Cryptococcus gattii and Cryptococcus neoformans. J Antimicrob Chemother. 2015;70:1396-407. https://doi.org/10.1093/jac/dku554
Venkateswarlu K, Taylor M, Manning NJ, Rinaldi MG, Kelly SL. Fluconazole tolerance in clinical isolates of Cryptococcus neoformans. Antimicrob Agents Chemother. 1997;41:748-51. https://doi.org/10.1128/AAC.41.4.748
Thornewell SJ, Peery RB, Skatrud PL. Cloning and characterization of CneMDR1: A Cryptococcus neoformans gene encoding a protein related to multidrug resistance proteins. Gene. 1997;201:21-9. https://doi.org/10.1016/s0378-1119(97)00421-6
Posteraro B, Sanguinetti M, Sanglard D, La Sorda M, Boccia S, Romano L, et al. Identification and characterization of a Cryptococcus neoformans ATP binding cassette (ABC) transporter-encoding gene, CnAFR1, involved in the resistance to fluconazole. Mol Microbiol. 2003;47:357-71. https://doi.org/10.1046/j.1365-2958.2003.03281.x
Sanguinetti M, Posteraro B, La Sorda M, Torelli R, Fiori B, Santangelo R, et al. Role of AFR1, an ABC transporter-encoding gene, in the in vivo response to fluconazole and virulence of Cryptococcus neoformans. Infect Immun. 2006;74:1352-9. https://doi.org/10.1128/IAI.74.2.1352-1359.2006
Espinel-Ingroff A, Aller AI, Canton E, Castañón-Olivares LR, Chowdhary A, Cordoba S, et al. Cryptococcus neoformans-Cryptococcus gattii species complex: An international study of wild-type susceptibility endpoint distributions and epidemiological cutoff values for fluconazole, itraconazole, posaconazole, and voriconazole. Antimicrob Agents Chemother. 2012;56:5898–906. https://doi.org/10.1128/AAC.01115-12
Clinical and Laboratory Standards Institute. Values TDIE. Epidemiological cutoff values for antifungal susceptibility testing. Fecha de consulta: 5 de octubre de 2022. Disponible en: https://clsi.org/media/1934/m59ed2_sample-updated.pdf
Procop GW, Alexander BD, Dufresne PJ, Fuller J, Ghannoum MA, Hanson KE, et al. Method for antifungal disk diffusion susceptibility testing of yeasts. Fecha de consulta: 5 de octubre de 2022. Disponible en: https://clsi.org/media/2634/m44ed3_sample.pdf
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402-8. https://doi.org/10.1006/meth.2001.1262
Gast CE, Basso LR, Bruzual I, Wong B. Azole resistance in Cryptococcus gattii from the Pacific Northwest: Investigation of the role of ERG11. Antimicrob Agents Chemother. 2013;57:5478-85. https://doi.org/10.1128/AAC.02287-12
Cannon RD, Lamping E, Holmes AR, Niimi K, Baret PV, Keniya MV, et al. Efflux-mediated antifungal drug resistance. Clin Microbiol Rev. 2009;22:291-321. https://doi.org/10.1128/CMR.00051-08
Li M, Liao Y, Chen M, Pan W, Weng L. Antifungal susceptibilities of Cryptococcus species complex isolates from AIDS and non-AIDS patients in Southeast China. Braz J Infect Dis. 2012;16:175-9. https://doi.org/10.1016/s1413-8670(12)70301-x
Wu SY, Kang M, Liu Y, Chen ZX, Xiao YL, He C, et al. Molecular epidemiology and antifungal susceptibilities of Cryptococcus species isolates from HIV and non-HIV patients in Southwest China. Eur J Clin Microbiol Infect Dis. 2021;40:287-95. https://doi.org/10.1007/s10096-020-04013-4
Bongomin F, Oladele RO, Gago S, Moore CB, Richardson MD. A systematic review of fluconazole resistance in clinical isolates of Cryptococcus species. Mycoses. 2018;61:290-7. https://doi.org/10.1111/myc.12747
Liu JY, Shi C, Wang Y, Li WJ, Zhao Y, Xiang MJ. Mechanisms of azole resistance in Candida albicans clinical isolates from Shanghai, China. Res Microbiol. 2015;166:153-61. https://doi.org/10.1016/j.resmic.2015.02.009
Florio AR, Ferrari S, De Carolis E, Torelli R, Fadda G, Sanguinetti M, et al. Genome-wide expression profiling of the response to short-term exposure to fluconazole in Cryptococcus neoformans serotype A. BMC Microbiol. 2011;11:97. https://doi.org/10.1186/1471-2180-11-97
Chang M, Sionov E, Khanal LA, Kwon-Chung KJ, Chang YC. Roles of Three Cryptococcus neoformans and Cryptococcus gattii efflux pump-coding genes in response to drug treatment. Antimicrob Agents Chemother. 2018;62:e01751-17. https://doi.org/10.1128/AAC.01751-17
Sionov E, Chang YC, Garraffo HM, Kwon-Chung KJ. Heteroresistance to fluconazole in Cryptococcus neoformans is intrinsic and associated with virulence. Antimicrob Agents Chemother. 2009;53:2804–15. https://doi.org/10.1128/AAC.00295-09
Some similar items:
- Jairo Lizarazo, Melva Linares, Catalina de Bedout, Ángela Restrepo, Clara Inés Agudelo, Elizabeth Castañeda, Grupo Colombiano para el Estudio de la Criptococosis, Results of nine years of the clinical and epidemiological survey on cryptococcosis in Colombia, 1997-2005 , Biomedica: Vol. 27 No. 1 (2007)
- Carolina Firacative, Germán Torres, María Claudia Rodríguez, Patricia Escandón, First environmental isolation of Cryptococcus gattii serotype B, from Cúcuta, Colombia , Biomedica: Vol. 31 No. 1 (2011)
- Catalina de Bedout, Julio Ayabaca, Ricardo Vega, Matilde Méndez, Axel R. Santiago, María Lucrecia Pabón, Angela Tabares, Myrtha Arango, Angela Restrepo, Vance Newell, Evaluation of Candida species' susceptibility to fluconazole with the disk diffusion method. , Biomedica: Vol. 23 No. 1 (2003)
- Patricia Escandón, Elizabeth Quintero, Diana Granados, Sandra Huérfano, Alejandro Ruiz, Elizabeth Castañeda, Isolation of Cryptococcus gattii serotype B from detritus of Eucalyptus trees in Colombia. , Biomedica: Vol. 25 No. 3 (2005)
- Sunny Sánchez, Dolores Zambrano, Maylen García, César Bedoya, Carlos Fernández, María Teresa Illnait-Zaragozí, Molecular characterization of Cryptococcus neoformans isolates from HIV patients, Guayaquil, Ecuador , Biomedica: Vol. 37 No. 3 (2017)
- Efraín Guillermo Sánchez , David Acosta , Juan Álvarez, Gabriela Sánchez , Julio García-Casallas, Disseminated cryptococcosis by biological therapy: We must manage the risk , Biomedica: Vol. 42 No. 2 (2022)
- Ana Elisa Rojas, Leidy Yurany Cárdenas, María Camila García, Jorge Enrique Pérez, Expression of ERG11, ERG3, MDR1 and CDR1 genes in Candida tropicalis , Biomedica: Vol. 43 No. Sp. 1 (2023): Agosto, Micología médica
- James Alexander Castillo , Natalia Afanasjeva, Method validation for the quantification of fluconazole and its organic impurities in raw material using high-performance liquid chromatography , Biomedica: Vol. 43 No. Sp. 1 (2023): Agosto, Micología médica
- Fernando Antonio Messina, Andrés Benchetrit, Andrea Bocassi, María de las Mercedes Romero, Sofía Bauer, Emmanuel Marín, Facundo Bertera, Guillermo Onis, Matías Enzenhofer, Milagro Sánchez, Lilia Mammana, Dana Mijalovsky, Gabriela Santiso, Meningeal cryptococcosis and SARS-CoV-2 infection in people living with HIV/AIDS , Biomedica: Vol. 43 No. Sp. 1 (2023): Agosto, Micología médica
Copyright (c) 2022 Biomedica

This work is licensed under a Creative Commons Attribution 4.0 International License.
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























