First report of the F1534C mutation associated with cross-resistance to DDT and pyrethroids in Aedes aegypti from Colombia
Abstract
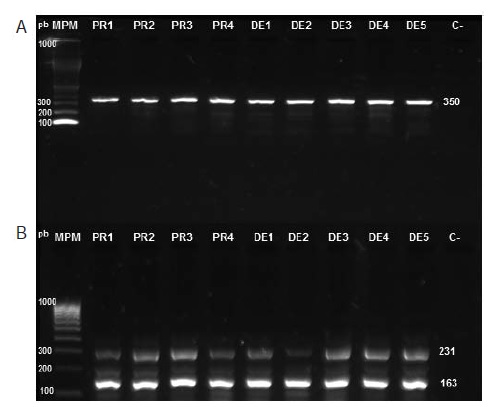
Introduction: The main strategy for the control of Aedes aegypti, vector of dengue, chikungunya and Zika viruses, is based on the use of insecticides to reduce its populations. However, their use has led to insect resistance to these chemicals. Objective: To determine the presence of the F1534C mutation associated with cross-resistance to DDT and pyrethroids in A. aegypti in Sincelejo, Colombia. Materials and methods: We studied nine specimens of A. aegypti that showed resistance to lambdacyhalothrin in bioassays developed by the Secretaría de Salud de Sucre. We used a semi-nested PCR as previously described by Harris, et al., to amplify exon 31 of the para gene of the voltage-dependent sodium channel of A. aegypti. We sequenced, edited, and analyzed PCR products with the MEGA 5 software. Results: We detected the wild and mutant alleles of exon 31 in all of the nine mosquitoes tested, and observed the substitution of thymine for guanine in the nucleotide sequence of the mutant allele, producing a change to UGC in the UUC codon, which led to the replacement of phenylalanine by cysteine in residue 1534 of the protein. Conclusion: The nine mosquitoes analyzed presented a heterozygote genotype for the F1534C mutation, whose phenotypic effect is knockdown resistance (kdr) to DDT and pyrethroids.
Downloads
References
Trujillo M, Marquetti M, Vásquez A, Montes J. Dinámica estacional y temporal de Aedes aegypti (Diptera: Culicidae) en el municipio Cienfuegos. Rev Cubana Med Trop. 2010;62:98-106.
World Health Organization. Guidelines for prevention and control of chikungunya fever. World Health Organization, Regional Office for South-East Asia, New Delhi: WHO; 2009. Fecha de consulta: 12 de septiembre de 2014. Disponible en: http://www.wpro.who.int/mvp/topics/ntd/Chikungunya_WHO_SEARO.pdf
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496:504-7. http://dx.doi.org/10.1038/nature12060
Instituto Nacional de Salud. Guía de atención clínica integral del paciente con dengue. Fecha de consulta: 20 de diciembre de 2014. Disponible en: http://www2.paho.org/col/dmdocuments/Guiadengue210310.pdf
Bisset JA, Rodríguez MM, San Martín JL, Romero JE, Montoya R. Evaluación de la resistencia a insecticidas de una cepa de Aedes aegypti de El Salvador. Rev Panam Salud Pública. 2009;26:229-34. http://dx.doi.org/10.1590/S1020-49892009000900007
Morrison AC, Zielinski-Gutiérrez E, Scott TW, Rosenberg R. Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Med. 2008;5:e68. http://dx.doi.org/10.1371/journal.pmed.0050068
Achee NL, Gould F, Perkins TA, Reiner RC, Morrison AC, Ritchie SA, et al. A critical assessment of vector control for dengue prevention. PLoS Negl Trop Dis. 2015;9:e0003655. http://dx.doi.org/10.1371/journal.pntd.0003655
van Den Berg H, Zaim M, Singh R, Soares A Ameneshewa B, Mnzava A, et al. Global trends in the use of insecticides to control vector-borne diseases. Environ Health Perspect. 2012;20:577-82. http://dx.doi.org/10.1289/ehp.1104340
Ranson H, Burhani J, Lumjuan N, Black WC. Insecticide resistance in dengue vectors. TropIKA.net. 2010;1:1-12.
Maestre R, Rey G, De Las Salas J, Vergara C, Santacoloma L, Goenaga S, et al. Estado de la susceptibilidad de Aedes aegypti a insecticidas en Atlántico (Colombia). Rev Colomb Entomol. 2010;36:242-8.
Dong K, Du Y, Rinkevich F, Nomura Y, Xu P, Wang L, et al. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem Mol Biol. 2014;50:1-17. http://dx.doi.org/10.1016/j.ibmb.2014.03.012
Brengues C, Hawkes NJ, Chandre F, Mccarroll L, Duchon S, Guillet P, et al. Pyrethroid and DDT cross-resistance in Aedes aegyptiis correlated with novel mutations in the voltage-gated sodium channel gene. Med Vet Entomol. 2003;13:87-94. http://dx.doi.org/10.1046/j. 1365-2915.2003.00412.x
Fonseca I, Quiñones ML. Resistencia a insecticidas en mosquitos (Diptera: Culicidae): mecanismos, detección y vigilancia en salud pública. Rev Colomb Entomol. 2005;31:107-15.
Anaya YP. Evaluación de la susceptibilidad a insecticidas en Aedes aegypti capturados en el municipio de Sincelejo, departamento de Sucre, Colombia (tesis). Sincelejo: Universidad de Sucre; 2008.
Maestre R, Gómez D, Ponce G, Flores AE. Susceptibility to insecticides and resistance mechanisms in Aedes aegypti from the Colombian Caribbean Region. Pestic Biochem Physiol. 2014;116:63-73. http://dx.doi.org/10.1016/j.pestbp. 2014.09.014
Mazzari M. Revisión del estado actual de la resistencia en Aedes aegypti a insecticidas utilizados en salud pública. Bol Mal Salud Amb. 1995;35:90-5.
Ocampo CB, Salazar MJ, Mina NJ, Mcallister J, Brogdon W. Insecticide resistance status of Aedes aegypti in 10 localities in Colombia. Acta Trop. 2011;118:37-44. http://dx. doi.org/10.1016/j.actatropica.2011.01.007
World Health Organization.Insecticide resistance and vector control: WHO; 1970. Fecha de consulta: 22 de agosto de 2014. Disponible en: http://apps.who.int/iris/bitstream/10665/40771/1/WHO_TRS_443_(part1).pdf
Caldera SM, Jaramillo MC, Cochero S, Pérez A, Bejarano EE. Diferencias genéticas entre poblaciones de Aedes aegypti de municipios del norte de Colombia, con baja y alta incidencia de dengue. Biomédica. 2013;33:89-98. http://dx. doi.org/10.7705/biomedica.v33i0.1573
Harris AF, Shavanthi R, Ranson H. Pyrethroid resistance in Aedes aegypti from Grand Cayman. Am J Trop Med Hyg. 2010;83:277-84. http://dx.doi.org/10.4269/ajtmh.2010. 09-0623
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28: 2731-9. http://dx.doi.org/10.1093/molbev/msr121
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Blast. Basic local alignment search tool. J Mol Biol. 1990;215:403-10. http://dx.doi.org/10.1016/S0022-2836(05)80360-2
Williamson MS, Martínez D, Hick CA, Devonshire AL. Identification of mutations in the housefly para-type sodium channel gene associated with knockdown resistance (kdr) to pyrethroid insecticides. Mol Gen Genet. 1996;252:51-60.
Aponte HA, Penilla RP, Dzul-Manzanilla F, Che-Mendoza A, López AD, Solís F, et al. The pyrethroid resistance status and mechanisms in Aedes aegypti from the Guerrero state, México. Pest Biochem Physiol. 2013;107:226-34. http://dx.doi.org/10.1016/j.pestbp.2013.07.005
Birggitt JG, Brito PL, Azambuja G, Saori A, Vieira R, Pereira JB, et al. Distribution and dissemination of the Val1016Ile and Phe1534Cys Kdr mutations in Aedes aegypti Brazilian natural populations. Parasit Vectors. 2014;7:25. http://dx.doi.org/10.1186/1756-3305-7-25
Kushwah RB, Dykes CL, Kapoor N, Adak T, Singh OP. Pyrethroid-resistance and presence of two knockdown resistance (kdr) mutations, F1534C and a novel mutation T1520I, in Indian Aedes aegypti. PLoS Negl Trop Dis. 2015;91:e3332. http://dx.doi.org/10.1371/journal.pntd. 0003332
Álvarez LC, Ponce G, Saavedra-Rodríguez K, López B, Flores AE. Frequency of V1016I and F1534C mutations in the voltage-gated sodium channel gene in Aedes aegypti in Venezuela. Pest Manag Sci. 2015;71:863-9. http://dx.doi.org/10.1002/ps.3846
Yanola J, Somboon P, Walton C, Nachaiwieng W, Prapanthadara L. A novel F1552/C1552 point mutation in the Aedes aegypti voltage-gated sodium channel gene associated with permethrin resistance. Pestic Biochem Physiol. 2010;96:127-31. http://dx.doi.org/10.1016/j.pestbp. 2009.10.005
Some similar items:
- Andrés Gómez-Palacio, Juan Suaza-Vasco, Sandra Castaño, Omar Triana, Sandra Uribe, Aedes albopictus (Skuse, 1894) infected with the American-Asian genotype of dengue type 2 virus in Medellín suggests its possible role as vector of dengue fever in Colombia , Biomedica: Vol. 37 No. Sup. 2 (2017): Suplemento 2, Entomología médica, 2017
- Juan Sebastián Sánchez, Ana María Cañón, Jadith Cristina Lombo, Subacute and chronic symptoms of chikungunya fever in a group of adults in Colombia , Biomedica: Vol. 39 No. 3 (2019)
- José Joaquín Carvajal, Nildimar Alves Honorio, Silvia Patricia Díaz, Edinso Rafael Ruiz, Jimmy Asprilla, Susanne Ardila, Gabriel Parra-Henao, Detection of Aedes albopictus (Skuse) (Diptera: Culicidae) in the municipality of Istmina, Chocó, Colombia , Biomedica: Vol. 36 No. 3 (2016)
- José Joaquín Carvajal, Ligia Inés Moncada, Mauricio Humberto Rodríguez, Ligia del Pilar Pérez, Víctor Alberto Olano, Characterization of Aedes albopictus (Skuse, 1894) (Diptera:Culicidae) larval habitats near the Amazon River in Colombia , Biomedica: Vol. 29 No. 3 (2009)
- Wilber Gómez-Vargas, Kelly Valencia-Jiménez, Guillermo Correa-Londoño, Faiber Jaramillo-Yepes, Novel larvicide tablets of Bacillus thuringiensis var. israelensis: Assessment of larvicidal effect on Aedes aegypti (Diptera: Culicidae) in Colombia , Biomedica: Vol. 38 No. Sup. 2 (2018): Suplemento 2, Medicina tropical
- Marcela Conde, Lorena I. Orjuela, Cesar Augusto Castellanos, Manuela Herrera-Varela, Susana Licastro, Martha L. Quiñones, Insecticide susceptibility evaluation in Aedes aegypti populations of Caldas, Colombia, in 2007 and 2011 , Biomedica: Vol. 35 No. 1 (2015)
- Myriam Lucía Velandia-Romero, Víctor Alberto Olano, Carolina Coronel-Ruiz, Laura Cabezas, María Angélica Calderón-Peláez, Jaime Eduardo Castellanos, María Inés Matiz, Dengue virus detection in Aedes aegypti larvae and pupae collected in rural areas of Anapoima, Cundinamarca, Colombia , Biomedica: Vol. 37 No. Sup. 2 (2017): Suplemento 2, Entomología médica, 2017
- Mauricio Hernández, Diana Arboleda, Stephania Arce, Allan Benavides, Paola Andrea Tejada, Sindy Vanessa Ramírez, Ángela Cubides, Methodology to develop endemic channels and notification trends for dengue in Valle del Cauca, Colombia, 2009-2013 , Biomedica: Vol. 36 (2016): Suplemento 2, Enfermedades virales
- Sandy Milena Caldera, María Cristina Jaramillo, Suljey Cochero, Alveiro Pérez-Doria, Eduar Elías Bejarano, Genetic differences between populations of Aedes aegypti from municipalities in northern Colombia, with high and low dengue incidence , Biomedica: Vol. 33 (2013): Suplemento 1, Fiebres hemorrágicas
- María Elena Cuéllar-Jiménez, Olga Lucía Velásquez-Escobar, Ranulfo González-Obando, Carlos Andrés Morales-Reichmann, Detection of Aedes albopictus (Skuse) (Diptera: Culicidae) in the city of Cali, Valle del Cauca, Colombia , Biomedica: Vol. 27 No. 2 (2007)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























