Genomic fragment detection and infectivity evaluation of rotaviruses isolated from wastewater used for irrigation in western Bogotá, D. C.
Abstract
Introduction. Enteric viruses significantly impact morbidity, mortality, and healthcare. Transmission through wastewater is favoured in highly contaminated areas due to inadequate treatment.
Objective. To determine the number of rotaviruses and their infectious capacity from wastewater samples used for irrigation in the western part of Bogotá.
Materials and methods. Concentrations of group A rotavirus were monitored in wastewater using molecular methods. The infectivity of rotaviruses was evaluated in a mouse intestinal villi model. We assessed the feasibility of applying this approach for environmental health surveillance in Colombia, considering findings reported by other authors.
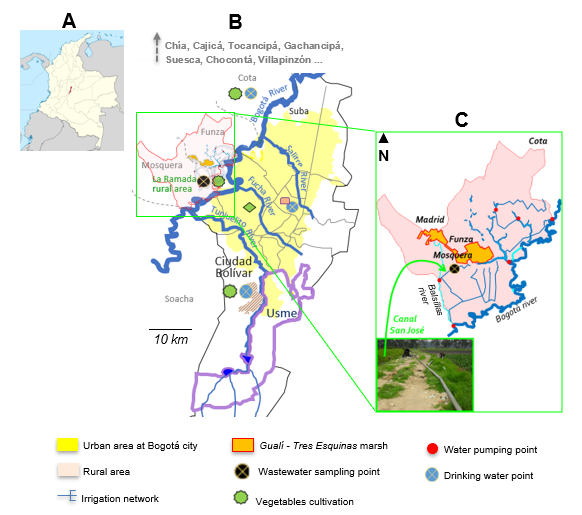
Results. The research focused on the La Ramada irrigation network in the western part of Bogotá, specifically the Canal San José. We analysed eighteen wastewater samples using qRT-PCR and detected group A rotavirus in twelve of them. The positive samples contained infectious rotavirus, as confirmed through the mouse villi model.
Conclusion. This study shows that contamination by group A rotavirus is frequent in wastewaters from the Canal San José in the La Ramada irrigation network in the western part of Bogotá and reveals high concentrations of rotavirus. The results suggest that villi from mouse intestines serve as a reliable model for isolating rotavirus from wastewaters. These findings provide a new approach for environmental health surveillance in Colombia, based on molecular epidemiology for waters highly contaminated with human enteric viruses.
Downloads
References
Randazzo W, Truchado P, Cuevas-Ferrando E, Simón P, Allende A, Sánchez G. SARSCoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181:1-8. https://doi.org/10.1016/j.watres.2020.115942
La Rosa G, Laconelli M, Mancini P, Bonanno Ferraro G, Veneri C, Bonadonna L, et al. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci Total Environ. 2020;736:1-5. https://doi.org/10.1016/j.scitotenv.2020.139652
Haramoto E, Malla B, Thakali O, Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci Total Environ. 2020;737:1-8. https://doi.org/10.1016/j.scitotenv.2020.140405
Teixeira P, Costa S, Brown B, Silva S, Rodrigues R, Valério E. Quantitative PCR detection of enteric viruses in wastewater and environmental water sources by the Lisbon municipality: A case study. Water. 2020;12:1-13. https://doi.org/10.3390/w12020544
Kobayashi N, Oshiki M, Ito T, Segawa T, Hatamoto M, Kato T, et al. Removal of human pathogenic viruses in a down-flow hanging sponge (DHS) reactor treating municipal wastewater and health risks associated with utilization of the effluent for agricultural irrigation. Water Res. 2017;110:389-98. https://doi.org/10.1016/j.watres.2016.10.054
La Rosa G, Pourshaban M, Iaconelli M, Muscillo M. Quantitative real-time PCR of enteric viruses in influent and effluent samples from wastewater treatment plants in Italy. Ann Ist Super Sanita. 2010;46:266-73. https://doi.org/10.4415/ann_10_03_07
Chen Y, Chen L, Deng Q, Zhang G, Wu K, Ni L, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833-40. https://doi.org/10.1002/jmv.25825
Chigor VN, Okoh AI. Quantitative RT-PCR detection of hepatitis A virus, rotaviruses and enteroviruses in the Buffalo river and source water dams in the Eastern Cape province of South Africa. Int J Environ Res Public Health. 2012;9:4017-32. https://doi.org/10.3390/ijerph9114017
Paranychianakis NV, Salgot M, Angelakis AN. Irrigation with recycled water: Guidelines and regulations. In: Levy GJ, Fine P, Bar-Tal A, editors. Treated wastewater in agriculture: Use and impacts on the soil environment and crops. Oxford: Blackwell Publishing Ltd; 2010. p. 77-112. https://doi.org/10.1002/9781444328561.ch3
Jordan-Lozano J. Human enteric pathogens circulation in the Bogotá region and its impact on the health of vulnerable communities [thesis]. Avignon: Université d’Avignon, Bogotá: Universidad Nacional de Colombia; 2021. Accessed: June 1 2025. Available at: https://theses.hal.science/tel-03464118
Ahmed W, Angel N, Edson J, Bibby K, Bivins A, O’Brien JW, et al. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: A proof of concept for the wastewater surveillance of COVID-19 in the community. Sci Total Environ. 2020;728:1-8. https://doi.org/10.1016/j.scitotenv.2020.138764
Jordan-Lozano JS. Las aguas residuales domésticas como alternativa de vigilancia epidemiológica del SARS-CoV-2 y otros virus entéricos humanos de potencial pandémico: una propuesta significativa para la salud pública en Colombia. Gest Ambien. 2021;24:94-9. https://doi.org/10.15446/ga.v24nSupl3.97597
Mallapaty S. How sewage could reveal true scale of coronavirus outbreak. Nature. 2020;580:176-7. https://doi.org/10.1038/d41586-020-00973-x
Daughton C. The international imperative to rapidly and inexpensively monitor communitywide COVID-19 infection status and trends. Sci Total Environ. 2020;726:1-2. https://doi.org/10.1016/j.scitotenv.2020.138149
Gobierno de Colombia. Plan Director Agua y Saneamiento Básico: Visión estratégica 2018-2030. Bogotá: Ministerio de Vivienda, Ciudad y Territorio. Accessed: June 12 2025. Available at: https://www.minvivienda.gov.co/sites/default/files/2020-07/plan-director.pdf
Rodríguez-Miranda JP, García-Ubaque CA, García-Ubaque JC. Enfermedades transmitidas por el agua y saneamiento básico en Colombia. Rev Salud Pública. 2016;18:738-45. https://doi.org/10.15446/rsap.v18n5.54869
Jordan JS, Mora CJ, Renault P, Guerrero CA. Mouse intestinal villi as a model system for studies of norovirus infection. Acta Virol. 2023;67:24-41. https://doi.org/10.4149/av_2023_103
Eitzinger A, Läderach P, Bunn C, Quiroga A, Benedikter A, Pantoja A, et al. Implications of a changing climate on food security and smallholders’ livelihoods in Bogotá, Colombia. Mitig Adapt Strateg Glob Chang. 2014;19:161-76. https://doi.org/10.1007/s11027-012-9432-0
Moreno AJ, Perdomo CA, Avilés OF. Study of climate change in Bogotá, using Colombia and global temperature data. Int J Appl Eng Res. 2018;13:11225-30.
Haramoto E, Katayama H, Oguma K, Ohgaki S. Application of cation-coated filter method to detection of noroviruses, enteroviruses, adenoviruses, and torque teno viruses in the Tamagawa River in Japan. Appl Environ Microbiol. 2005;71:2403-11. https://doi.org/10.1128/AEM.71.5.2403-2411.2005
Guerrero CA, Santana AY, Acosta O. Mouse intestinal villi as a model system for studies of rotavirus infection. J Virol Methods. 2010;168:22-30. https://doi.org/10.1016/j.jviromet.2010.04.010
Schmitz BW, Kitajima M, Campillo ME, Gerba CP, Pepper IL. Virus reduction during advanced Bardenpho and conventional wastewater treatment processes. Environ Sci Technol. 2016;50:9524-32. https://doi.org/10.1021/acs.est.6b01384
Kitajima M, Iker BC, Pepper IL, Gerba CP. Relative abundance and treatment reduction of viruses during wastewater treatment processes — Identification of potential viral indicators. Sci Total Environ. 2014;488-489:290-6. https://doi.org/10.1016/j.scitotenv.2014.04.087
González MM, Sarmiento L, Castaño JC, Giraldo AM, Salazar A, Nini Y, et al. Detección de poliovirus en aguas residuales de Armenia, Colombia. Rev Salud Pública. 2006;8:13-23.
Hewitt J, Leonard M, Greening GE, Lewis GD. Influence of wastewater treatment process and the population size on human virus profiles in wastewater. Water Res. 2011;45:6267-76. https://doi.org/10.1016/j.watres.2011.09.029
Le Cann P, Ranarijaona S, Monpoeho S, Le Guyader F, Ferré V. Quantification of human astroviruses in sewage using real-time RT-PCR. Res Microbiol. 2004;155:11-5. https://doi.org/10.1016/j.resmic.2003.09.013
Tesson V, Renault P. Reversible immobilization and irreversible removal of viruses in soils or mixtures of soil materials; an open data set enriched with a short review of main trends. HAL open science. Accessed: June 11 2025. Available from: https://hal.science/hal-01655017/document
Haramoto E, Katayama H, Ohgaki S. Detection of noroviruses in tap water in Japan by means of a new method for concentrating enteric viruses in large volumes of freshwater. Appl Environ Microbiol. 2004;70:2154-60. https://doi.org/10.1128/AEM.70.4.2154-2160.2004
Zamora-Figueroa A, Rosales RE, Fernández R, Ramírez V, Bastardo M, Farías A, et al. Detection and diversity of gastrointestinal viruses in wastewater from Caracas, Venezuela, 2021-2022. Virology. 2024;589:1-12. https://doi.org/10.1016/j.virol.2023.109913
Picciola Bordoni G, Gonçalves Barbosa LC, Santos Lima F, de Oliveira Santos M, Gonçalves Vieira JD, Oliveira TR, et al. Rotavirus group A occurrence in rural water source samples in a midwest region state of Brazil, comparing wet and dry seasons. Viruses. 2024;16:1-10. https://doi.org/10.3390/v16091452
Some similar items:
- María Fernanda Gutiérrez, Sandra Moreno, Mónica Viviana Alvarado, Andrea Bermúdez, DNA sequence analysis indicates human origin of rotavirus and hepatitis A virus strains from western Colombia , Biomedica: Vol. 29 No. 2 (2009)
- Martha N. Calderón, Carlos Guerrero, Yohana Domínguez, Eliana Garzón, Sandra M. Barreto, Orlando Acosta, Interaction of rotavirus with protein disulfide isomerase in vitro and cell system , Biomedica: Vol. 31 No. 1 (2011)
- Dioselina Peláez, Blanca Lisseth Guzmán, Johanna Rodríguez, Felipe Acero, Gerardo Nava, Presence of enteric viruses in water samples for consumption in Colombia: Challenges for supply systems , Biomedica: Vol. 36 (2016): Suplemento 2, Enfermedades virales
Copyright (c) 2025 Biomedica

This work is licensed under a Creative Commons Attribution 4.0 International License.
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |
Funding data
-
Fondation Nestlé
Grant numbers Reference 45/2016

























