Facial nerve injuries cause changes in central nervous system microglial cells
Abstract
Introduction: Our research group has described both morphological and electrophysiological changes in motor cortex pyramidal neurons associated with contralateral facial nerve injury in rats. However, little is known about those neural changes, which occur together with changes in surrounding glial cells.
Objective: To characterize the effect of the unilateral facial nerve injury on microglial proliferation and activation in the primary motor cortex.
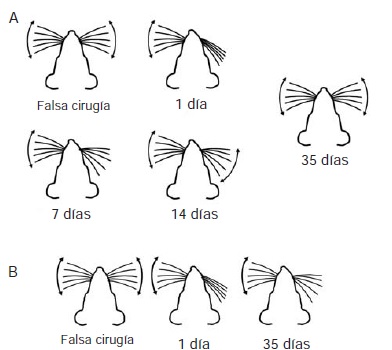
Materials and methods: We performed immunohistochemical experiments in order to detect microglial cells in brain tissue of rats with unilateral facial nerve lesion sacrificed at different times after the injury. We caused two types of lesions: reversible (by crushing, which allows functional recovery), and irreversible (by section, which produces permanent paralysis). We compared the brain tissues of control animals (without surgical intervention) and sham-operated animals with animals with lesions sacrificed at 1, 3, 7, 21 or 35 days after the injury.
Results: In primary motor cortex, the microglial cells of irreversibly injured animals showed proliferation and activation between three and seven days post-lesion. The proliferation of microglial cells in reversibly injured animals was significant only three days after the lesion.
Conclusions: Facial nerve injury causes changes in microglial cells in the primary motor cortex. These modifications could be involved in the generation of morphological and electrophysiological changes previously described in the pyramidal neurons of primary motor cortex that command facial movements.
Downloads
References
Brecht M, Preilowsky B, Merzenich M. Functional archi-tecture of the mystacial vibrissae. Behav Brain Res. 1997;84: 81-97. http://dx.doi.org/10.1016/S0166-4328(97)83328-1
Carvell G, Simmons D. Biometric analyses of vibrissae tactile discrimination in the rat. J Neurosci. 1990;10:2638-48.
Mehta SB, Kleinfeld D. Frisking the whiskers: Patterned sensory input in the rat vibrissa system. Neuron. 2004;41: 181-4. http://dx.doi.org/10.1016/S0896-6273(04)00002-9
Carvell GE, Simons DJ, Lichtenstein SH, Bryant P. Electromyographic activity of mystacial pad musculature during whisking behavior in the rat. Somatosens Mot Res. 1991;8:159-64.
Berg RW, Kleinfeld D. Vibrissa movement elicited by rhythmic electrical microstimulation to motor cortex in the aroused rat mimics exploratory whisking. J Neurophysiol. 2003;90:2950-63. http://dx.doi.org/10.1152/jn.00511.2003
Hattox AM, Priest CA, Keller A. Functional circuitry involved in the regulation of whisker movements. J Comp Neurol. 2002;442:266-76. http://dx.doi.org/10.1002/cne.10089
Grinevich V, Brecht M, Osten P. Monosynaptic pathway from rat vibrissa motor cortex to facial motor neurons revealed by lentivirus-based axonal tracing. J. Neurosci. 2005;25:8250-8. http://dx.doi.org/10.1523/JNEUROSCI. 2235-05.2005
Izraeli R, Porter LL. Vibrissal motor cortex in the rat: Connections with the barrel field. Exp Brain Res. 1995;104: 41-54. http://dx.doi.org/10.1007/BF00229854
Moran LB, Graeber MB. The facial nerve axotomy model. Brain Res Brain Res Rev. 2004;44:154-78. http://dx.doi.org/10.1016/j.brainresrev.2003.11.004
Brännström T, Kellerth JO. Recovery of synapses in axotomized adult cat spinal motoneurons after reinnervation into muscle. Exp Brain Res. 1999;125:19-27. http://dx.doi.org/10.1007/s002210050653
Peyghambari F, Valojerdi MR, Tiraihi T. A morphometric study on the early stages of dendrite changes in the axotomized motoneuron of the spinal cord in newborn rats. Neurol Res. 2005;27:586-90. http://dx.doi.org/10.1179/ 016164105X48743
Gustafsson B. Changes in motoneurone electrical pro-perties following axotomy. J Physiol. 1979;293:197-215. http://dx.doi.org/10.1113/jphysiol.1979.sp012885
Laiwand R, Werman R, Yarom Y. Electrophysiology of degenerating neurones in the vagal motor nucleus of the guinea-pig following axotomy. J Physiol. 1988;404:749-66. http://dx.doi.org/10.1113/jphysiol.1988.sp017317
Mentis GZ, Díaz E, Morán LB, Navarrete R. Early alterations in the electrophysiological properties of rat spinal motoneurones following neonatal axotomy. J Physiol. 2007;582:1141-61. http://dx.doi.org/10.1113/jphysiol.2007. 133488
Yan Q, Matheson C, López OT, Miller JA. The biological responses of axotomized adult motoneurons to brain-derived neurotrophic factor. J Neurosci. 1994;14:5281-91.
Kobayashi NR, Bedard AM, Hincke MT, Tetzlaff W. Increased expression of BDNF and trkB mRNA in rat facial motoneurons after axotomy. Eur J Neurosci. 1996;8:1018-29. http://dx.doi.org/10.1111/j.1460-9568.1996.tb01588
Haas CA, Donath C, Kreutzberg GW. Differential expres-sion of immediate early genes after transection of the facial nerve. Neurosci. 1993;53:91-9. http://dx.doi.org/10. 1016/0306-4522(93)90287-P
Schmitt AB, Breuer S, Liman J, Buss A, Schlangen C, Pech K, et al. Identification of regeneration-associated genes after central and peripheral nerve injury in the adult rat. BMC Neurosci. 2003;4:8-20. http://dx.doi.org/10. 1186/1471-2202-4-8
Urrego D, Múnera A, Troncoso J. Retracción a largo plazo del árbol dendrítico de neuronas piramidales córtico-faciales por lesiones periféricas del nervio facial. Biomédica. 2011;31:560-9. http://dx.doi.org/10.7705/biomedica.v31i4.440
Urrego D, Troncoso J, Múnera A. Layer 5 pyramidal neurons’ dendritic remodeling and increased microglial density in primary motor cortex in a murine model of facial paralysis. Biomed Res Int. 2015;2015:482023. http://dx.doi.org/10.1155/2015/482023
Múnera A, Cuestas DM, Troncoso J. Peripheral facial nerve lesions induce changes in the firing properties of primary motor cortex layer 5 pyramidal cells. Neuroscience. 2012;223:140-51. http://dx.doi.org/10.1016/j.neuroscience. 2012.07.063
Laskawi R, Rohlmann A, Landgrebe M, Wolff JR. Rapid astroglial reactions in the motor cortex of adult rats following peripheral facial nerve lesions. Eur Arch Otorhinolaryngol. 1997;254:81-5. http://dx.doi.org/10.1007/BF01526185
Franchi G. Time course of motor cortex reorganization following botulinum toxin injection into the vibrissal pad of the adult rat. Eur J Neurosci. 2002;16:1333-48. http://dx.doi.org/10.1046/j.1460-9568.2002.02195.x
Rappert A, Bechmann I, Pivneva T, Mahlo J, Biber K, Nolte C, et al. CXCR3-dependent microglial recruitment is essential for dendrite loss after brain lesion. J Neurosci. 2004;24:8500-9. http://dx.doi.org/10.1523/JNEUROSCI. 2451-04.2004
Shokouhi BN, Wong BZ, Siddiqui S, Lieberman AR, Campbell G, Tohyama K, et al. Microglial responses around intrinsic CNS neurons are correlated with axonal regeneration. BMC Neurosci. 2010;11:13. http://dx.doi.org/10.1186/1471-2202-11-13
Luo XG, Chen SD. The changing phenotype of microglia from homeostasis to disease. Transl Neurodegener. 2012;1:9. http://dx.doi.org/10.1186/2047-9158-1-9
Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461-553. http://dx.doi.org/10.1152/physrev.00011.2010
Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6thedition. London, UK: Academic Press; 2007.
Raposo C, Schwartz M. Glial scar and immune cell involvement in tissue remodeling and repair following acute CNS injuries. Glia. 2014;62:1895-904. http://dx.doi.org/10.1002/glia.22676
Streit WJ, Graeber MB, Kreutzberg GW. Functional plasticity of microglia: A review. Glia. 1988;1:301-7. http://dx. doi.org/10.1002/glia.440010502
Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974-80. http://dx.doi.org/10. 1523/JNEUROSCI.4363-08.2009
Some similar items:
- Juan Carlos Miguel, Ariana Erazo, Fernanda Beduino, Juan Carlos Picena, María Isabel Luciano, Gustavo Pizzuti, María Cristina Tarrés, Silvana Montenegro, Stella Maris Martínez, Chronic bronchial dilatations in different colonies of laboratory rats , Biomedica: Vol. 22 No. 2 (2002)
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |

























